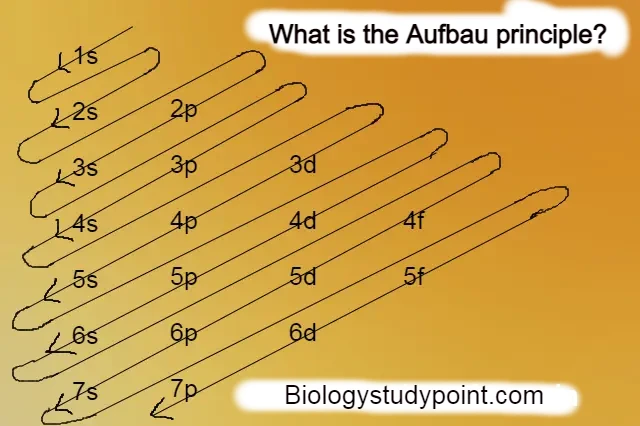
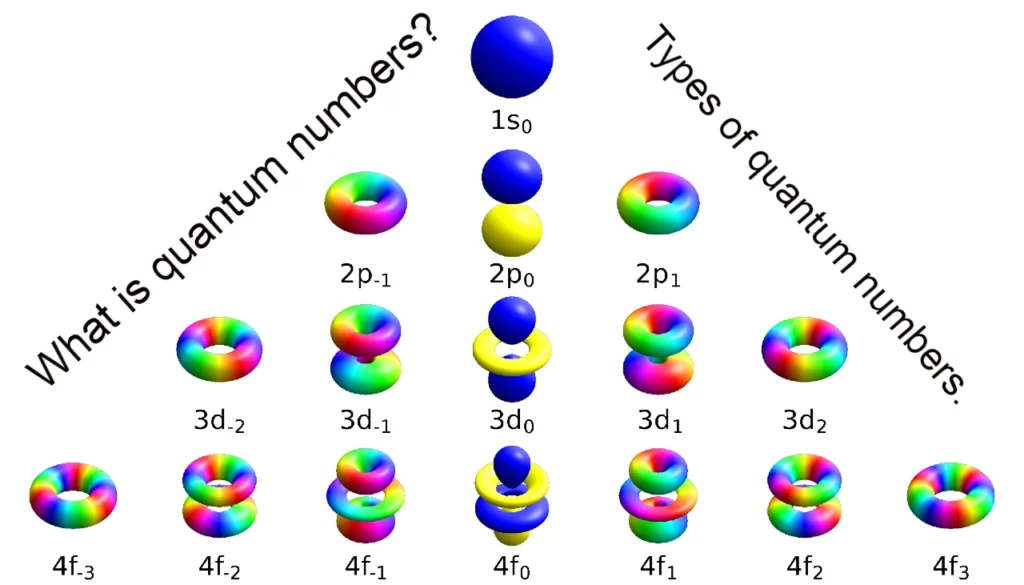
Hello friends, today in this article we will talk about atoms, what is an atom called? What is the principle of the atom? What is the atomic number called? Who discovered the atom? What is the atomic mass called? To learn about all these steps by step, then let’s start.
What is an atom?
An atom is the smallest particle of an element that is not found in the free state. For example, atoms of gold, silver, platinum, etc. take part in chemical reactions. But it does not split in chemical reactions. It is the smallest particle of the element – Cl, H, N, etc.
Dalton’s Atomic Model –
In 1808, a scientist named John Dalton presented his ideas regarding the atomic structure. This is called Dalton’s atomism. According to this principle, all the properties of matter are inherent in an atom.
| Dalton’s Atomic Model | Modern Atomic Model |
| An atom is the smallest particle of a particle. Which cannot be divided. | Atoms can be divided into fundamental particles like electrons, protons, and neutrons. |
| Atoms can neither be created nor destroyed. | It can be broken up by nuclear fission. |
| Compounds are formed by the combination of atoms. | Molecules are formed by the combination of atoms. |
| The same element has the same atomic mass. | The atomic mass of the same element can be different. So such elements are called isotopic. |
| Atoms of different elements have different masses. | Atoms of different elements can have the same mass. Such elements are called isobars. |
Who discovered the atom?
The atom was discovered by the scientist John Dalton. He was the first to tell about the atom.
Also, read – What is Atomic structure?
What is the atomic number?
Atomic number –
The atomic number is the fundamental or fundamental property of an atom or an element, which expresses the number of protons present in its nucleus or the total number of electrons present in its orbitals.
In other words, the total number of protons present in the nucleus of an element is called the atomic number. The total number of electrons is called the atomic number.
Because the total number of protons present in the nucleus of an element is equal to the total number of electrons.
What is called atomic mass?
Atomic Mass –
The atomic mass of an element is a number that shows how many times an atom of that element is one-twelfth the mass of an atom of carbon or 1.008 parts by mass of hydrogen. This number represents the ratio of the average mass of one atom of the element to one-twelfth the mass of an atom of carbon.
The atomic mass of the element = Mass of one atom of the element / Mass of one-twelfth of carbon (C12)
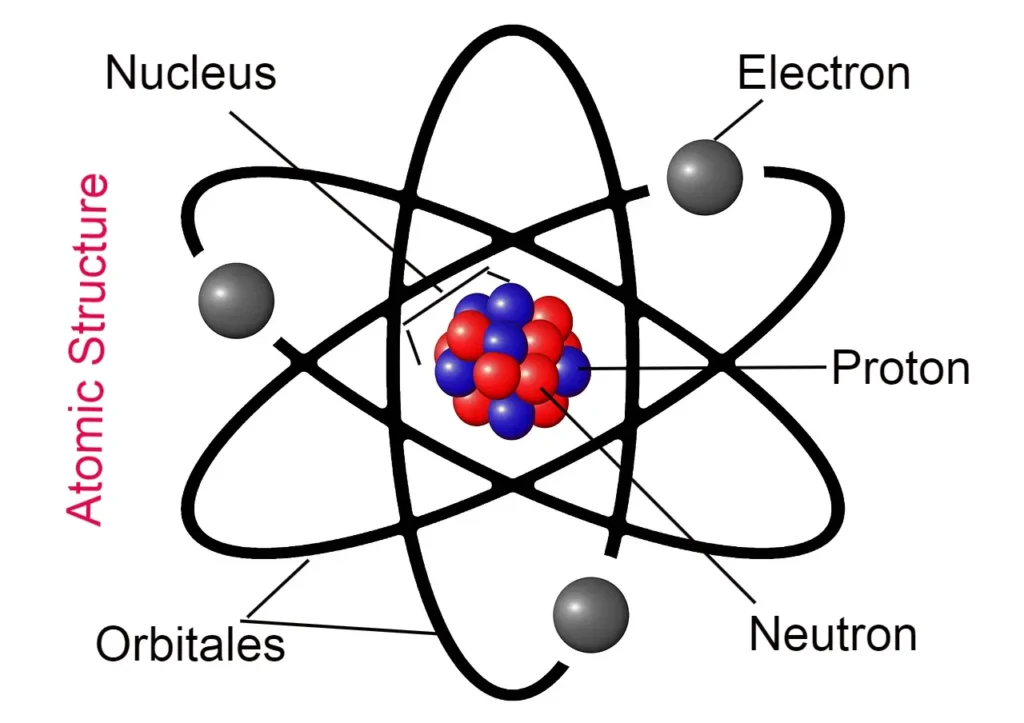
What are the fundamental particles of an atom called?
What are the fundamental particles of an atom? Or what are atoms made up of?
Fundamental Particles of Atom –
Those particles of an atom, which are the main components of the structure of the atom or together make up an atom. The fundamental particles of an atom are called.
The names of the fundamental particles of an atom –
- Electron
- Proton
- Neutron
What is an electron called?
Electrons are found in orbitals outside the atomic nucleus. It is the unit negatively charged particle of each atom. The charge of the electron is -1.603 x 10-19 coulomb or -4.808 x 10-10 e.s.u. it happens. The mass of an electron is 9.1095 x 10-28 grams. Its radius is 2.8 x 10-13 cm. It is represented by -1e0 or e.
Who discovered the electron?
The electron was discovered by scientist J.J. Thomson (1897).
What is Proton?
Protons are present in the atomic nucleus. It is the unit positively charged particle of each atom. The charge of the proton is +1.603 x 10-19 coulomb or +4.808 x 10-10 e.s.u. it happens. The mass of a proton is 1.6726 x 10-24 grams. Its radius is 10-13 cm, denoted by 1H1 or p of the proton.
Who discovered the proton?
The proton was discovered by the scientist E. Rutherford (1919).
What is a Neutron?
Neutrons are also found in the atomic nucleus, but there is no charge found on it and it is neutral.
Who discovered the neutron?
The neutron was discovered by scientist J. Chadwick (1932).
I hope, friends, who do you call nuclear? Would have liked the information given about What is an Atom. Friend if you like this information. So please share it with your friends so that they can also benefit from it.
Thank you so much