Do you want to study Chemistry from scratch? If yes, then this article is exclusively for you because today we are going to study the very first chapter of Chemistry class 11th, “Some Basic Concepts of Chemistry,” in straightforward language. So let’s start without wasting any time.
Definition of Chemistry
Chemistry is The branch of science that studies changes that occur in substances during their composition, properties, structures, and chemical reactions.
How did the word Chemistry originate?
The word Chemistry originated from the word Chemia, which means the black color.
Who is the father of Chemistry?
Antoine Laurent de Lavoisier is called the father of Chemistry.
Significance of Chemistry
The importance of Chemistry is in all fields. There is no field where chemical substances are not used. Some fields include agriculture, medicine, food substances, pesticides, construction materials, health, clothing, plastics and glass, cosmetics, fuel, specific substance synthesis, environmental pollution control, metals, and alloys, etc.
How many parts are there in Chemistry?
There are several parts in Chemistry, but its main three parts are:
- Physical Chemistry: Under this, we study the Chemistry part involving calculations.
- Inorganic Chemistry: Under this, we study inorganic compounds like H2O, HCl, H2SO4, etc.
- Organic Chemistry: Under this, we study organic substances like methane, ethane, propane, etc.
Matter/Substance
The substance that occupies some space and has some mass is called matter.
Classification of Matter
We will classify matter based on two criteria:
- Physical Classification
- Chemical Classification
Physical Classification
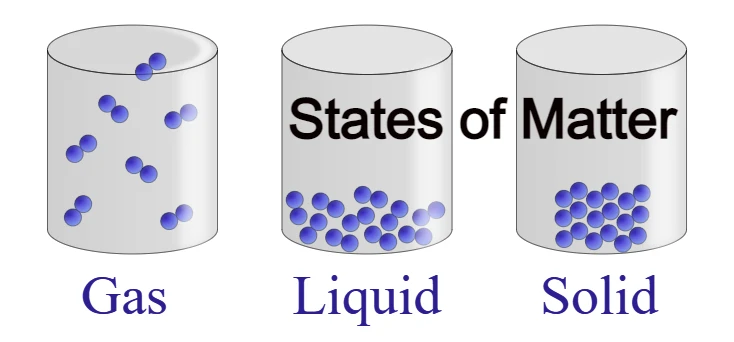
It is divided based on states. The following are included: solid, liquid, gas, plasma, and Bose-Einstein Condensate.
Solid – Its shape and volume are definite because its particles are closely packed. Its attraction force is strong. Examples include wood, brick, pen, etc.
Liquid – Its volume is definite but its shape is indefinite because its particles are a bit apart. That means it takes the shape of the container it is put in. Its attraction force is moderate. Examples include water, oil, etc.
Gas – Both its shape and volume are indefinite because its particles are far apart and keep moving. That is, it spreads completely in the container it is put in.
Plasma – When a gas is heated to very high temperatures, its atoms release electrons and become ionized, and this state of matter is called plasma. It is found in the Sun, stars, etc. because there the temperature is very high.
Bose-Einstein Condensate – This is the fifth state of matter. Around 1924, Indian scientist Satendra Nath Bose researched and wrote a letter to the scientist Einstein, who read and studied that letter or research and described the fifth state of matter, which is called Bose-Einstein Condensate. This state is achieved when a very low-density gas is cooled down, resulting in this state. Suppose the density of a gas is 1, then when its density becomes one millionth, this state is achieved.
Chemical Classification
Based on this, it is divided into two parts: Pure substance and Impure substance or Mixture.
Pure Substance
When the constituent particles of a substance are chemically the same, it is called a pure substance. Or, all those substances in the world that have a chemical formula are kept in pure substances according to Chemistry.
It includes two substances:
- Element
- Compound
Element – The smallest particle that cannot be divided into simpler forms by any physical or chemical process is called an element. Elements are made up of the same type of atoms. The fundamental particle of any element is an atom.
Examples – H, He, N, C, Li, etc.
It is divided into three categories:
- Metal – Na, Ag, Fe, Cu, Zn, etc.
- Non-metal – N2, F2, Cl2, O2, P4, S8, etc.
- Metalloid – Si, As, Te, At, Ge, Sb, Po, etc.
Compound – A substance that is made up of two or more elements and has a definite ratio of the constituent elements is called a compound. Its basic particle is an atom.
Examples – HCl, NaCl, H2SO4, etc.
Atoms combine to form molecules and elements combine to form compounds.
How many types of compounds?
There are two types:
Organic Compounds –
Those compounds in which carbon and hydrogen bonds or chains are found are called organic compounds.
Examples – sucrose, glucose, methane, and other hydrocarbons.
Inorganic Compounds –
Those compounds in which carbon and hydrogen bonds or chains are not found are known inorganic compounds.
Examples – HCl, H2O, NaCl, K2SO4, etc.
Mixture –
When two or more elements or substances are mixed in any proportion, it is called a mixture, or all those substances in the world that do not have a chemical formula are kept in impure substances or mixtures according to Chemistry. It is divided into two parts.
- Homogeneous Mixture
- Heterogeneous Mixture
Homogeneous Mixture
A mixture in which the arrangement of components is the same everywhere is called a homogeneous mixture. Examples include seawater, air, alloys, 22-carat gold, colored stones, salt water, sugar water, etc.
Heterogeneous Mixture –
A mixture in which the arrangement of components is not uniform everywhere is called a heterogeneous mixture. Examples include milk, wood, water + sand, clouds, etc.
Properties of matter and their measurement
Properties of matter can be of two types:
Physical Properties –
All those properties that do not change the chemical composition of a substance are called physical properties. These include taste, odor, texture, boiling point, melting point, state, color, etc.
Chemical Properties –
Whenever something reacts with other chemical agents like acids, bases, hydrogen, water, oxygen, halogens, air, salts, alcohols, etc., it is called a chemical property.
Measurement of Physical Properties
International System of Units (SI).
| Physical quantity | symbol for quantity | SI unit name | SI unit symbol |
|---|---|---|---|
| length | l | meter | m |
| mass | m | kilogram | kg |
| time | t | second | s |
| electric current | I | ampere | A |
| thermodynamic temperature | T | kelvin | K |
| amount of substance | n | mole | mol |
| luminous intensity | lv | candela | cd |
Prefixes used in the SI system
| Multiple | Prefix | Symbol |
|---|---|---|
| 10-24 | yocto | y |
| 10-21 | zepto | z |
| 10-18 | atto | a |
| 10-15 | femto | f |
| 10-12 | pico | p |
| 10-9 | nano | n |
| 10-6 | micro | μ |
| 10-3 | milli | m |
| 10-2 | centi | c |
| 10-1 | deci | d |
| 10 | deca | da |
| 102 | hecto | h |
| 103 | kilo | k |
| 106 | mega | M |
| 109 | giga | G |
| 1012 | tera | T |
| 1015 | peta | P |
| 1018 | exa | E |
| 1021 | zeta | Z |
| 1024 | yotta | Y |
Physical Properties of Matter –
Mass (kg), Weight (N), Volume (m3), Density (kg/m3), Temperature (K)
International System of Units (SI) for Measurements
MKS – Length (m), Mass (kg), Time (s).
SI – cm, gm, s
What is Mass and Weight?
Definition of Mass –
The amount of matter present in a substance is known as its mass, which remains constant and does not change. For example, carbon, nitrogen, sodium, lithium, etc., all these elements have some mass, as the atomic number of carbon is 6, and its atomic mass is 12 which remains constant.
Weight –
The weight of an object that changes with its position or location is called weight.
For example, when a person goes to the moon, their weight becomes 1/6th because the gravitational force there is less, so the weight also decreases.
Volume –
The space occupied by an object is called its volume. When we measure the volume enclosed by an object, we express it in terms of length, width, and height, always in m3.
Density –
The mass of a substance present in a unit volume is called its density.
Density = Mass/Volume
= Kg/m3
= gm/cm3
Temperature –
Temperature is divided into three parts:
- Centigrade (℃)
- Kelvin (K)
- Fahrenheit (℉)
Formula –
℃ = K – 273
K = ℃ + 273
℉ = 9/5 x ℃ + 32
Q. Convert 373 Kelvin to Fahrenheit.
℃ = K – 273
℃ = 373 – 273 = 100
Formula: ℉ = 9/5 * ℃ + 32
℉ = 9/5 * 100 + 32
℉ = 180 + 32
℉ = 212
Define Uncertainty in Measurement.
In this, we study accuracy and precision.
Definition of Accuracy –
The value which is close to each other but not close to the actual value, such values are called accuracy. For example, assuming the actual value is 2.00 kg. The measurement is coming first time 1.93 kg and the second time 1.95 kg.
The value which is not close to each other nor close to the actual value, such values are neither accurate nor precise. For example – 1.94 kg and 1.97 kg
Definition of Precision –
The value which is close to each other and also close to the actual value, such values are called accuracy and precision. For example – 1.99 and 2.01 kg.
What is Scientific Notation?
When a number is written in the form of “N×10n“, it is called scientific notation.
Formula – N×10n ( N will be a number)
Ex. 254×102 is not a large number but if 436728 is a large number then we write it in scientific notation like this 4.36728×105 and if 0.0036 is such a number then we write it in scientific notation like this 3.6×10-3
Practice –
Q. How can we write the following numbers in scientific notation?
234.405, 356700, 0.0005
Solution –
234.405 = 2.34405×105
356700 = 3.56700×105
0.0005 = 5.0×10-4
What are Significant Figures?
The number of digits used in expressing a physical quantity accurately is called significant figures.
For example, in 0.007, there is one significant figure.
When 0 (zero) is before a number, it is not significant but when 0 is after or between a number, it is significant.
Examples –
- 407 here ‘0’ is not significant. There are 3 significant figures.
- 2.04 here also 0 is not significant. There are also 3 significant figures.
- 2.200 here also 0 is not significant. There are 4 significant figures.
- 001 here zero is not significant and there is one significant figure.
- 259.607 There are six significant figures.
If numbers are written like this, how will we write significant figures?
2.0×102 = two significant figures.
2.00×102 = three significant figures.
3.000×102 = four significant figures.
Among these, the most stable significant figure is 2.00×102 because numbers up to two digits after the decimal are considered significant.
Read next