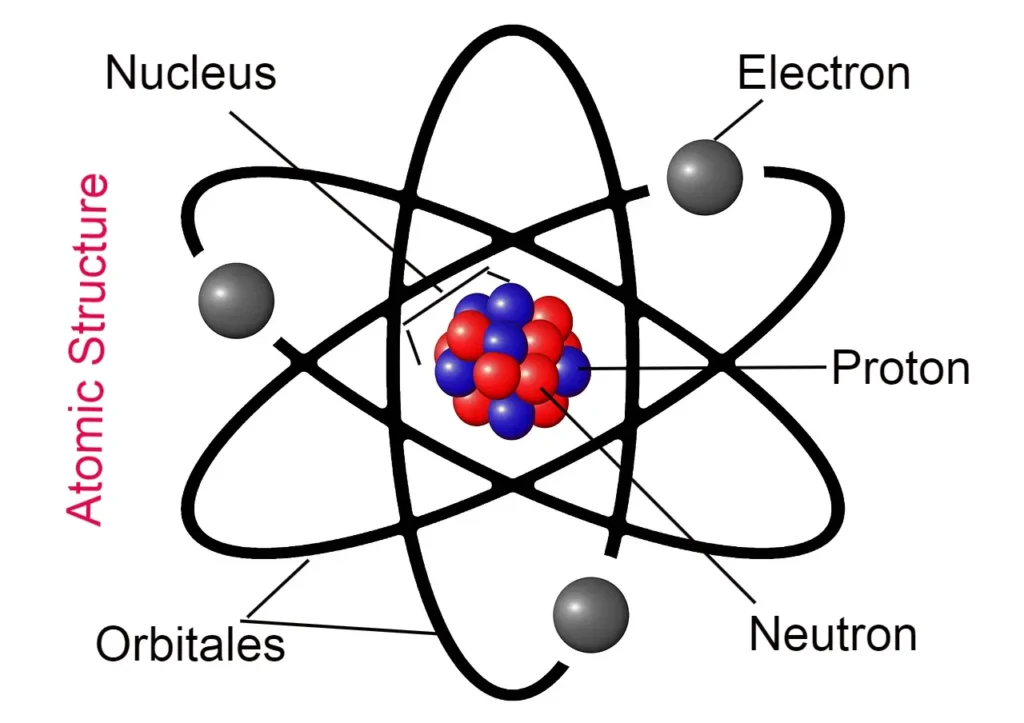
Hello friends, would you like to study John Dalton’s atomic theory in a very easy language? If yes, then this article is for you because, in this article, we will study atomic theory, the definition of Dalton’s law, flaws in the theory, atomic mass, average atomic mass, etc. So let’s start without wasting time.
John Dalton’s Atomic Theory
In the year 1808, scientist John Dalton proposed a theory based on several experiments.
- The matter is made up of microscopic particles called atoms.
- All atoms of a single element are identical in size, shape, and mass, while atoms of different elements differ in these properties.
- Atoms cannot be created or destroyed.
- Atoms of different elements combine in simple whole-number ratios to form compounds.
- Atoms of different elements can have different atomic masses and sizes.
Dalton’s law is defined as follows.
Dalton’s law states that each matter is made up of tiny particles. An atom cannot be divided chemically or physically.
Flaws in Dalton’s Atomic Theory
- According to this theory, atoms are indivisible particles, but they are made up of three particles (electrons, protons, neutrons), which means atoms can be divided.
- According to this theory, all atoms of an element have the same atomic mass and size, but due to isotopes, all atoms of the same element can have different atomic masses and slightly different sizes. For example, hydrogen isotopes.
- According to this theory, the atomic masses of atoms of different elements are different, but after the discovery of isotopes, it was found that atoms of different elements can have the same atomic mass.
- This theory does not explain Galois-Sacc’s law of combining volumes.

Atomic Mass:
The mass of one atom is called atomic mass, and its unit is the unified atomic mass unit (u).
Examples – 12C6, 13C6, 14C6
1H1 or P (Protium), 2H1 or D (Duterium), 3H1 or T (Tritium)
Average Atomic Mass:
The average atomic mass is the weighted average of the atomic masses of isotopes of an element found in nature.
Average Atomic Mass = Sum of isotopes’ mass × abundance of isotopes / Total number of isotopes
12+13+14/3 = 13
Molecular Mass:
The sum of the atomic masses of all atoms in a molecule is the molecular mass. It is expressed in unified atomic mass units (u).
For example,
H2O = 2H + O
H2O = 2✕1 + 16 = 18
H2SO4 = 2✕1 + 32 + 4✕16 = 98
CH4 = 12 + 4✕1 = 16
The next heading of this chapter will be provided in the next part. If you liked this article, please share it in your Facebook and Whatsapp groups so that more people can benefit. Thank you.