“Would you like to start learning about the laws of chemical combination from the beginning? If yes, then this article is exclusively for you because today we will study the heading ‘Laws of Chemical Combination‘ from the first chapter of chemistry class 11th in elementary language. So let’s start without wasting any time.
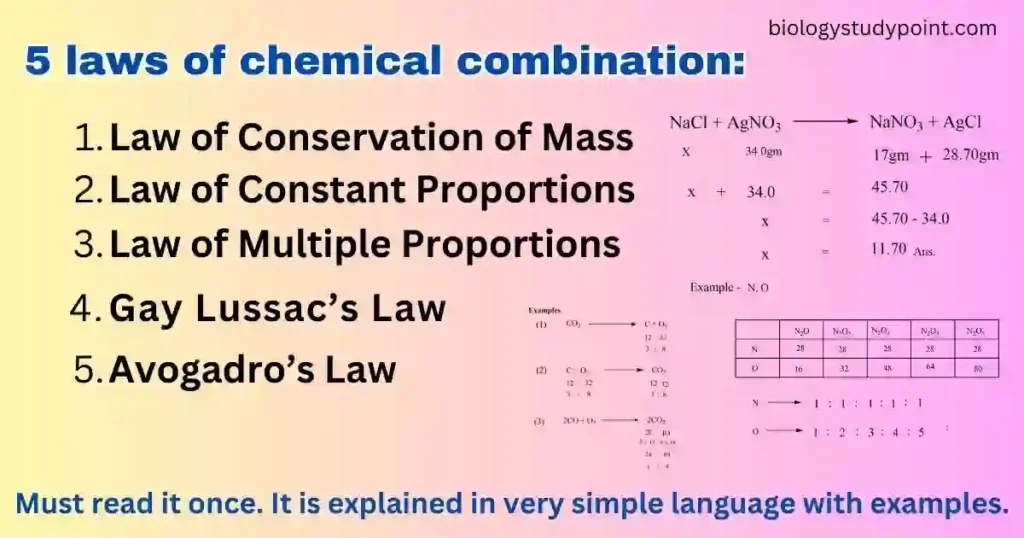
What are the 5 laws of chemical combination:
These are the rules that chemical reactions follow to occur. What are these rules?
- Law of Conservation of Mass
- Law of Constant Proportions
- Law of Multiple Proportions
- Gay Lussac’s Law
- Avogadro’s Law
Law of Conservation of Mass:
In any reaction, the total mass of reactants equals the total mass of products, meaning there is no increase or decrease in mass. This is called the law of conservation of mass.
This law was first proposed by Antoine Lavoisier, known as the father of chemistry.
Example:
CH4 + 2O2 → CO2 + 2H2O
2H2 + O2 → 2H2O
Law of Constant Proportions:
Definition – According to this law, elements in a chemical substance are always present in fixed mass ratios. Scientist Joseph Proust proposed this law.
For example, no matter where water is taken from, be it a waterfall, lake, pond, stream, river, etc., its ratio remains 1:8.
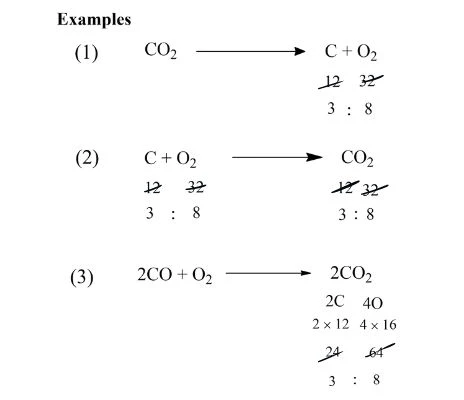
Q. If 4 grams of hydrogen react with some oxygen to form 36 grams of water, using the law of conservation of mass, how much oxygen was used?
Ans.
Hydrogen + Oxygen → Water
Given Mass: Hydrogen = 4 grams, Oxygen = x grams
Water = 36 grams
According to the law of conservation of mass:
Mass of reactants = Mass of products
4 grams + x grams = 36 grams
x grams = 36 grams – 4 grams
x grams = 32 gm Answer
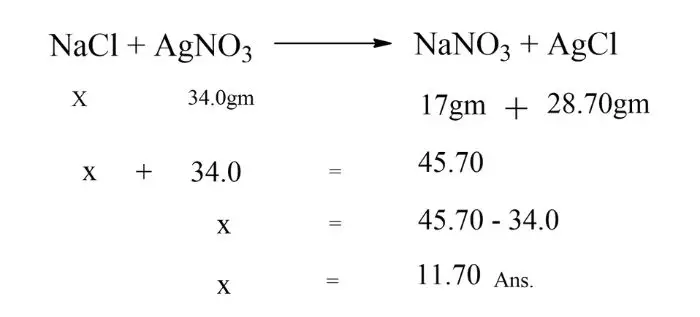
Q. If the law of conservation of mass is true, how much sodium chloride will be produced by reacting 34.0 grams of sodium chloride with 17 grams of sodium nitrate and 28.70 grams of silver chloride?
Q. According to which rule is it true that in a given compound there is always the same volume of elements according to weight?
Ans. Law of Constant Proportions
Law of Multiple Proportions:
Definition – If two elements can combine to form more than one compound, the masses of the elements in these compounds are in simple ratios. This law was proposed by John Dalton.
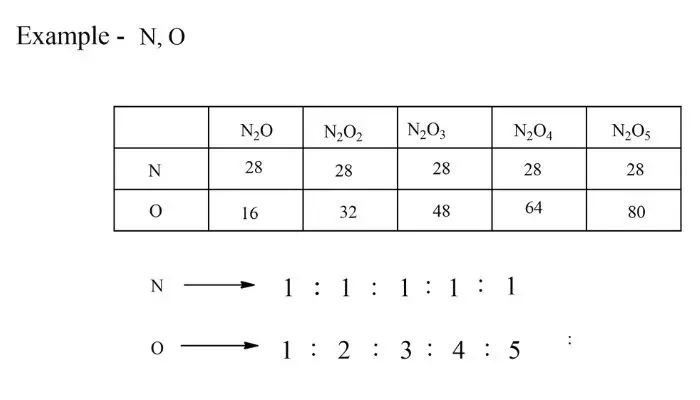
Gay Lussac’s Law:
When gases combine in chemical reactions, their volumes are in simple ratios provided all gases are at the same temperature and pressure.
Example – One volume of molecule H2 combines with one volume of Cl2 molecule to produce 2 volumes of undivided gas HCl.
Example:
2H2 + Cl2 → 2HCl
Volume 1L + 1L = 2L
N2 + 3H2 → 2NH3
1:3:2
Avogadro’s Law:
At the same temperature and pressure, the number of atoms in equal volumes of gases is the same. Initially, this relationship was called Avogadro’s hypothesis, but later it became known as Avogadro’s principle, and now it is called Avogadro’s law.
Important Points:
- The number of atoms in one gram of any gas is called Avogadro’s number.
- The value of Avogadro’s number is obtained from various methods and is 6.022 × 1023. It is denoted by N.
- The number of particles (atoms, molecules, ions) present in one mole of a substance is called Avogadro’s number.
Example 1:
The atomic weight of carbon C is 12, so one mole of carbon = 12 grams of carbon.
Thus, in 12 grams of carbon, the number of carbon atoms will be 6.022 × 1023.
Example 2:
The atomic weight of hydrogen H2 is 18.
So, one mole of water = 18 grams of water
Thus, in 18 grams of water, the number of water molecules will be 6.022 × 1023.
Q. What is the total number of particles in 34 grams of NH3?
1 mol NH3 = 17gm
Number of NH3 molecules = 6.022 × 1023
Number of NH3 molecules in 1gm = 6.022 × 1023/17
Number of NH3 molecules in 34gm = 6.022 × 1023× 34/17 = 12.04 × 1023
NH3 contains 1 N atom and 3 H atoms, so a total of 4 atoms.
Therefore, in 34 grams of NH3, the total number of particles = 4 × 12.04 × 1023 = 48.04 × 1023 Answer
Read next